CE marking
From CEOpedia | Management online
| CE marking |
|---|
| See also |
CE marking located on a product certifies that product meets the requirements set out in New Approach Directives of the European Union. The requirements apply particularly to safety, health and environmental users. CE marking means that the manufacturer or importer of a product meets the basic requirements of ESR (Essential Requirements). ERS are due to the requirements of the relevant Directives.
Steps which have to be taken to mark product with CE
To mark the CE product is necessary to take the following steps:
- Determine which directives apply,
- Determine the harmonized standards and basic requirements for health and safety that are applicable for a given product,
- Determine and select the notified body (if such a requirement poses a directive or if assistance and support are needed),
- Determine the appropriate compatibility assessment procedure,
- Make a product assessment,
- Attach the technical documentation,
- Prepare a declaration of compliance,
- Mark product with CE. The manufacturer (or a person authorized by him) has the task of place the CE marking on the product. In addition to the CE mark on a product should be placed the notified body number which made the assessment.
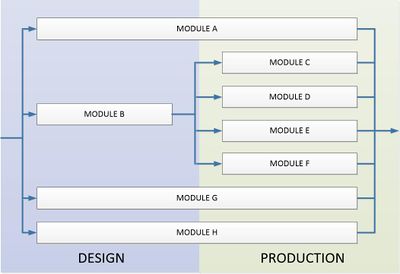
Modules for conformity assessment procedures
There are eight modules that apply to the design and production. Modules are different from each other in stages of development of products, mode of assessment and of a performance assessment.
- Module A - Declaration of conformity - internal control of production (design and manufacturing),
- Module B - Type test - a notified body issue a certificate of examination about the design stage complemented by module on the evaluation on the manufacture,
- Module C - Type compatibility - used with module B, manufacturer declares that the products concerned conform with the type described in the certificate of Type compatibility,
- Module D - Production quality assurances - used with module B, this module requires the participation of a notified body, which should approve and control the quality system of production, final inspection and testing,
- Module E - Product quality assurances - used with module B, this module requires the participation of a notified body, which should approve and control the quality system of final inspection and testing,
- Module F - Product verification - used with module B, the notified body shall oversee compliance with the certificate issued with module B, and issue a certificate of conformity,
- Module G - Verification of unit production - the notified body checks individual products and then issue a certificate of conformity,
- Module H - Full quality assurance - the notified body approves and oversees a quality system for design, manufacture, final inspection and testing
References
- CE marking on European Commission website
- Sedlacek G, Muller Ch (2006) The European standard family and its basis, Journal of Constructional Steel Research, Volume 62, Issue 11
- French-Mowat E, Burnett J (2012) How are medical devices regulated in the European Union?, Journal of the Royal Society of Medicine, 105:1
Author: Edyta Gołąb